Expanded ring N-heterocyclic carbene adducts of group 15 element trichlorides: synthesis and reduction studies†
Abstract
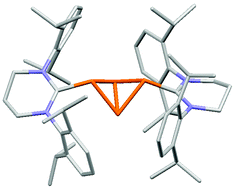
Reactions of the expanded ring N-heterocyclic carbene, 6-Dip (:C{N(Dip)CH2}2CH2, Dip = 2,6-diisopropylphenyl), with group 15 element trichlorides have yielded the monomeric complexes, [(6-Dip)ECl3] (E = P, As or Sb), two examples of which (E = P and Sb) have been crystallographically characterised. Reduction of [(6-Dip)PCl3] with KC8 yielded the unusual tetraphosphorus dicationic complex, [(6-Dip)2(μ-P4)]Cl2, the X-ray crystal structure of which shows it to be an ion-separate salt. The compound can also be prepared from the direct reaction of excess 6-Dip with PCl3. Treatment of the cyclic amidinium salt, [6-MesH]Br (6-MesH = [HC{N(Mes)CH2}2CH2]+, Mes = mesityl) with KC8, leads to reductive coupling of the heterocycle and formation of the hindered bis(hexahydropyrimidine), (6-MesH)2. An X-ray crystallographic analysis of (6-MesH)2 shows the compound to have a long central C–C bond, while an electrochemical analysis reveals it to undergo an irreversible two-electron oxidation in dichloromethane solutions.

 Please wait while we load your content...
Please wait while we load your content...