The bonding situation in triethylchalcogenostiboranes – polarized single bonds vs. double bonds†
Abstract
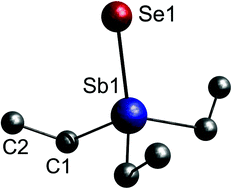
Triethylchalcogenostiboranes Et3Sb![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) E (E = S 1, Se 2) were synthesized and their solid state structures were determined. The Sb–Se bond length is the shortest ever reported. Short Sb⋯E contacts were not observed. According to quantum chemical calculations, the bonding situation in 1 and 2 is best described as a polarized Sb–E single bond.
E (E = S 1, Se 2) were synthesized and their solid state structures were determined. The Sb–Se bond length is the shortest ever reported. Short Sb⋯E contacts were not observed. According to quantum chemical calculations, the bonding situation in 1 and 2 is best described as a polarized Sb–E single bond.

 Please wait while we load your content...
Please wait while we load your content...