Boron-centered soft ligands based on tetrazole units and their complexes with sodium, potassium and bismuth ions†
Abstract
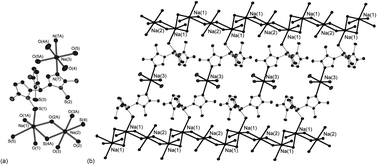
The syntheses of Na(TttMe) (1), K(TttMe) (2), K(BttMe) (3), and Na(BttPh) (4) are reported, where (TttMe)− and (BttR)− (R = Me, Ph) are tri- and di-substituted boron-centered soft ligands; they were produced by the reaction of NaBH4 and KBH4 with the corresponding tetrazole heterocycles: 1-methyl-5-thiotetrazole (L1H) and 1-phenyl-5-thiotetrazole (L2H). The syntheses of Na(TttMe) (1) and K(TttMe) (2) were carried out following Trofimenko's protocol. The anion (TttMe)− is a typical Janus scorpionate ligand. Na(TttMe) (1) and Na(BttPh) (4) were reacted with Bi(CH3COO)3 to observe the coordination pattern of these ligands towards bismuth ions. These reactions afforded the complexes [Bi(TttMe)2(CH3COO)] (5), [Bi(L2)2]2 (6a, 6b) and [Bi(L2)3] (7). The products were characterized by NMR spectroscopy, elemental analyses and mass spectrometry. Solid state structures were determined by X-ray diffraction of single crystals of 1, 2, 3, 4, 5, 6a, 6b and 7. M(TttMe) (M = Na (1), K (2)) and K(BttMe) (3) exhibit sheet-like structures. The alkali metal complexes 1–4 dissociate in solution as observed in 1H, 13C NMR and 1H diffusion NMR experiments. Negative mode ESI-MS data also indicate the presence of monomers. Both the sodium salts of (TttMe)− and (BttPh)− as well as the Bi complexes 5 and 7 were investigated by absorption and emission spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...