Synthesis and structural investigation of R2Si (R = Me, Ph) bridged di-N-heterocyclic carbenes†
Abstract
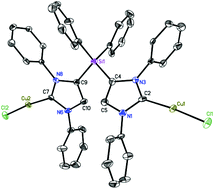
Functionalization of the C4 carbon of an imidazol-derived N-heterocyclic carbene (NHC) may allow fine-tuning of the electronic and steric properties of the C2 carbene center. A facile route to silyl-functionalized di-N-heterocyclic carbenes (Di-NHCs) is described. Treatment of the polymeric lithiated NHC, {Li(IPrH)}n (1) (Li(IPrH) = {(N-2,6-iPr2C6H3)2CHCLi}C:) with a dichlorosilane affords monomeric silyl-functionalized Di-NHCs, R2Si(IPrH)2 (R = Ph, 2; Me, 3). Interestingly, silyl-functionalized mono-NHC, Ph2(Cl)Si(IPrH) (4) with a pendant chloro-substituent can also be exclusively isolated maintaining the reactants 1 and Ph2SiCl2 ratio. NHCs 2 and 4 readily form copper complexes, Ph2Si{(IPrH)CuCl}2 (5) and Ph2(Cl)Si{(IPrH)CuCl} (6), on reaction with CuCl. Straightforward conversion of an NHC to a Di-NHC (2 or 3) via C4 functionalization is reported for the first time. Molecular structures of 2, 4, 5 and 6 have been established by single crystal X-ray diffraction studies.


 Please wait while we load your content...
Please wait while we load your content...