Design and development of POCN-pincer palladium catalysts for C–H bond arylation of azoles with aryl iodides†
Abstract
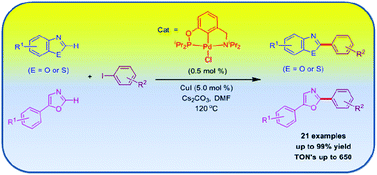
Well-defined and efficient POCN-ligated palladium complexes have been developed for the direct C–H bond arylation of azoles with aryl iodides. The phosphinite-amine pincer ligands 1-(R2PO)-C6H4-3-(CH2NiPr2) [R2POCNiPr2-H; R = iPr (1a), R = tBu (1b)] and corresponding palladium complexes {2-(R2PO)-C6H3-6-(CH2NiPr2)}PdCl [(R2POCNiPr2)PdCl; R = iPr (2a), R = tBu (2b)] were synthesized in good yields. Treatment of palladium complex 2a with KI and AgOAc afforded the complexes (iPr2POCNiPr2)PdI (3a) and (iPr2POCNiPr2)Pd(OAc) (4a), respectively. Similarly, the reaction of 2a with benzothiazolyl-lithium produces the (iPr2POCNiPr2)Pd(benzothiazolyl) (5a) complex in a quantitative yield. The pincer palladium complex 2a efficiently catalyzes the C–H bond arylation of benzothiazole, substituted-benzoxazoles and 5-aryl oxazoles with diverse aryl iodides in the presence of CuI as a co-catalyst under mild reaction conditions. This represents the first example of a pincer palladium complex being applied for the direct C–H bond arylation of any heterocycle with low catalyst loading. A preliminary mechanistic investigation reveals that palladium nanoparticles are presumably not the catalytically active form of 2a and supports the direct involvement of the catalyst 2a, with complex 5a being a probable key intermediate in the catalytic reaction.

 Please wait while we load your content...
Please wait while we load your content...