Nickel promoted functionalization of CO2 to anhydrides and ketoacids†
Abstract
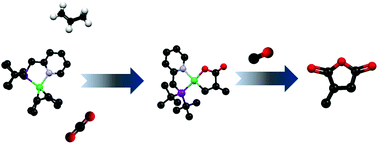
The reductive functionalization of carbon dioxide into high value organics was accomplished via the coupling with carbon monoxide and ethylene/propylene at a zerovalent nickel species bearing the 2-((di-t-butylphosphino)methyl)pyridine ligand (PN). An initial oxidative coupling between carbon dioxide, olefin, and (PN)Ni(1,5-cyclooctadiene) afforded five-membered nickelacycle lactone species, which were produced with regioselective 1,2-coupling in the case of propylene. The propylene derived nickelacycle lactone was isolated and characterized by X-ray diffraction. Addition of carbon monoxide, or a combination of carbon monoxide and diethyl zinc to the nickelacycle lactone complexes afforded cyclic anhydrides and 1,4-ketoacids, respectively, in moderate to high yields. The primary organometallic product of the transformation was zerovalent (PN)Ni(CO)2.

 Please wait while we load your content...
Please wait while we load your content...