Fluorescent Au(i)@Ag2/Ag3 giant cluster for selective sensing of mercury(ii) ion†
Abstract
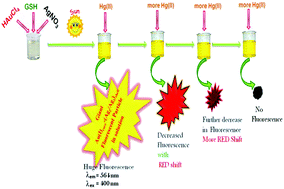
Highly stable Au(I)core–Ag(0)shell particles have been synthesized in aqueous solution via a green chemistry pathway utilising sunlight irradiation. The shell of the particles is composed of fluorescent Ag2 and Ag3 clusters which make the large core–shell particles highly fluorescent. The Au(I) core of the particles offers long-term stability to the silver clusters, which are otherwise unstable in solution at room temperature, by the transfer of electron density from the shell. Successive additions of Hg(II) ions to the fluorescent solution cause efficient and selective quenching of the fluorescence with gradual red shifting of the emission peak. The metallophilic 5d10(Hg2+)–4d10(Agδ+) interaction as well as Hg(II) stimulated aggregation have been ascribed to causing the fluorescence quenching and red shift. The fluorescent Au(I)core–Ag(0)shell particles are a highly selective and sensitive sensing platform for the detection of Hg(II) down to 6 nM in the presence of various metal ions. The detection limit is far below the permissible level as determined by the EPA. Interferences due to Cu(II) and Fe(III) have been eliminated using Na2-EDTA and NH4HF2, respectively. The fluorescent particles are successfully transferred to various solvent systems making Hg(II) determination also possible in non-aqueous media. Finally, the temperature dependent fluorescence change with and without Hg(II) provides information about the metallophilic interaction.

 Please wait while we load your content...
Please wait while we load your content...