Isoquinoline-derivatized tris(2-pyridylmethyl)amines as fluorescent zinc sensors with strict Zn2+/Cd2+ selectivity†
Abstract
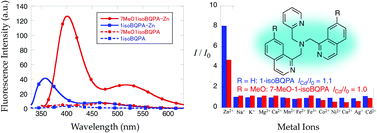
Tris(2-pyridylmethyl)amine-based fluorescent ligands, N,N-bis(1-isoquinolylmethyl)-2-pyridylmethylamine (1-isoBQPA) and N,N-bis(7-methoxy-1-isoquinolylmethyl)-2-pyridylmethylamine (7-MeO-1-isoBQPA), have been prepared and the Zn2+-induced fluorescence enhancement has been investigated. Upon excitation at 324 nm, 1-isoBQPA exhibits a very weak emission (ϕ = ∼0.010) in DMF–H2O (1 : 1). Upon Zn2+ addition, the 1-isoBQPA fluorescence increases (ϕZn = 0.055) at 357 nm and 464 nm. The fluorescence enhancement at longer wavelengths is Zn2+-specific, whereas Cd2+ induces a small emission increase at 464 nm (ICd/I0 = 1.1, ICd/IZn = 14%). The Zn2+/Cd2+ selectivity of the fluorescent response correlates with the Cd–Nisoquinoline and Zn–Nisoquinoline bond distances measured in the crystal structures. Introduction of methoxy groups into the 1-isoBQPA chromophore enhances the fluorescence significantly (ϕZn = 0.213), which affords 7-MeO-1-isoBQPA properties amenable for fluorescence microscopy in living cells.

 Please wait while we load your content...
Please wait while we load your content...