Computational and experimental study on the electrocatalytic reduction of CO2 to CO by a new mononuclear ruthenium(ii) complex†
Abstract
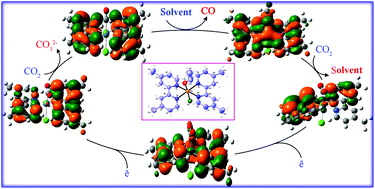
A new mononuclear ruthenium(II) complex, trans-[Ru(dmb)2(Cl)(EtOH)](PF6) (dmb = 4,4′-dimethyl-2,2′-bipyridine), has been prepared and characterized by elemental analysis, spectroscopic techniques and single crystal X-ray structure determination. The complex was studied as a precatalyst for the electrocatalytic reduction of CO2 to CO in an acetonitrile solution by cyclic voltammetry (CV). The catalytic mechanism was investigated by means of quantum chemical calculations to gain deeper insight into the process of CO2 reduction. The results suggest that the reaction proceeds in six steps initiating by the two sequential 1ē reductions at the dmb ligands followed by CO2 addition to give a metallocarboxylate intermediate. This intermediate undergoes further reduction and loses a CO molecule. The results reported in this paper are of great significance in providing theoretical insight into a class of electrocatalysts for reduction of CO2 to CO.

 Please wait while we load your content...
Please wait while we load your content...