Crystal structures and magnetic properties of a set of dihalo-bridged oxalamidato copper(ii) dimers†
Abstract
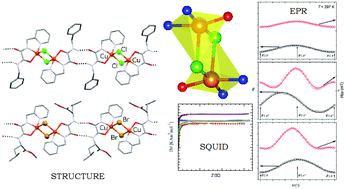
A set of four copper(II) complexes, L1–X and L2–X (X = Cl, Br; L1 = N-(L-leucine methyl ester)-N′-((2-pyridin-2-yl)methyl)oxalamide and L2 = N-benzyl-N′-((2-pyridin-2-yl)methyl)oxalamide), have been synthesized and characterized by X-ray structural analysis, electron paramagnetic resonance (EPR) spectroscopy on single crystals and by SQUID magnetization measurements. X-ray diffraction studies show one-dimensional hydrogen bonded networks of dimeric copper(II)-complexes bridged by two halide ions and with the two metal centers 3.44–3.69 Å apart. The geometry at each copper(II) atom is ideal or near ideal square pyramidal. EPR and SQUID studies indicate that all complexes exhibit weak antiferromagnetic interactions between the Cu(II) paramagnetic centers, with exchange parameter |J| ∼ 1 cm−1. Magneto-structural comparisons among similar dihalo-bridged Cu(II) dinuclear complexes are also provided, and a possible correlation has been established.

 Please wait while we load your content...
Please wait while we load your content...