Cytochrome P450-catalyzed dealkylation of atrazine by Rhodococcus sp. strain NI86/21 involves hydrogen atom transfer rather than single electron transfer†
Abstract
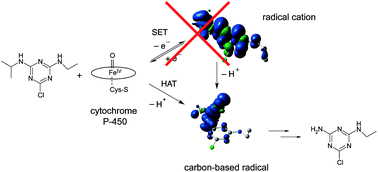
Cytochrome P450 enzymes are responsible for a multitude of natural transformation reactions. For oxidative N-dealkylation, single electron (SET) and hydrogen atom abstraction (HAT) have been debated as underlying mechanisms. Combined evidence from (i) product distribution and (ii) isotope effects indicate that HAT, rather than SET, initiates N-dealkylation of atrazine to desethyl- and desisopropylatrazine by the microorganism Rhodococcus sp. strain NI86/21. (i) Product analysis revealed a non-selective oxidation at both the αC and βC-atom of the alkyl chain, which is expected for a radical reaction, but not SET. (ii) Normal 13C and 15N as well as pronounced 2H isotope effects (εcarbon: −4.0‰ ± 0.2‰; εnitrogen: −1.4‰ ± 0.3‰, KIEH: 3.6 ± 0.8) agree qualitatively with calculated values for HAT, whereas inverse 13C and 15N isotope effects are predicted for SET. Analogous results are observed with the Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O model system [5,10,15,20-tetrakis(pentafluorophenyl)porphyrin-iron(III)-chloride + NaIO4], but not with permanganate. These results emphasize the relevance of the HAT mechanism for N-dealkylation by P450.
O model system [5,10,15,20-tetrakis(pentafluorophenyl)porphyrin-iron(III)-chloride + NaIO4], but not with permanganate. These results emphasize the relevance of the HAT mechanism for N-dealkylation by P450.

 Please wait while we load your content...
Please wait while we load your content...