Differences in the cyclometalation reactivity of bisphosphinimine-supported organo-rare earth complexes†
Abstract
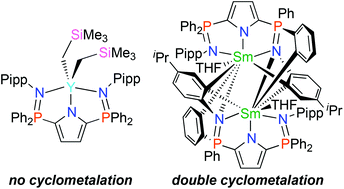
The pyrrole-based ligand N,N′-((1H-pyrrole-2,5-diyl)bis(diphenylphosphoranylylidene))bis(4-isopropylaniline) (HLB) can be deprotonated and coordinated to yttrium and samarium ions upon reaction with their respective trialkyl precursors. In the case of yttrium, the resulting complex [LBY(CH2SiMe3)2] (1) is a Lewis base-free monomer that is remarkably resistant to cyclometalation. Conversely, the analogous samarium complex [LBSm(CH2SiMe3)2] is dramatically more reactive and undergoes rapid orthometalation of one phosphinimine aryl substituent, generating an unusual 4-membered azasamaracyclic THF adduct [κ4-LBSm(CH2SiMe3)(THF)2] (2). This species undergoes further transformation in solution to generate a new dinuclear species that features unique carbon and nitrogen bridging units [κ1:κ2:μ2-LBSm(THF)]2 (3). Alternatively, if 2 is intercepted by a second equivalent of HLB, the doubly-ligated samarium complex [(κ4-LB)LBSm] (4) forms.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...