α-Borylcarbonyl compounds: from transient intermediates to robust building blocks
Abstract
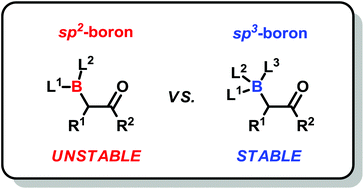
α-Borylcarbonyl species (C-bound boron enolates) are typically unstable due to their kinetic and thermodynamically favourable rearrangement to their O-bound isomers. Direct evidence of α-borylcarbonyl compounds is sparse despite the multitude of transformations in which they have been implicated as reactive intermediates. In recent years, a few examples have emerged of reactive α-borylcarbonyl intermediates that can be observed using spectroscopic methods. Other reports have shown that certain compounds containing the α-borylcarbonyl motif can be isolated under ambient conditions. Installation of electron-rich, tetracoordinate sp3-boron centers is a particularly viable strategy to improve the stability of α-borylcarbonyl systems as it imposes a barrier to the otherwise rapid 1,3-boron shift from carbon to oxygen. Among stable α-borylcarbonyl compounds, α-boryl aldehydes, equipped with a tetracoordinated N-methyliminodiacetyl (MIDA) boryl group, have been demonstrated to be versatile building blocks in the synthesis of a wide range of functionalized organoboron compounds.

 Please wait while we load your content...
Please wait while we load your content...