Uranyl-oxo coordination directed by non-covalent interactions†
Abstract
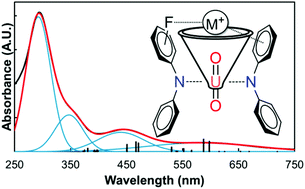
Directed coordination of weakly Lewis acidic K+ ions to weakly Lewis basic uranyl oxo ligands is accomplished through non-covalent cation–π and cation–F interactions for the first time. Comparison of a family of structurally related diarylamide ligands highlights the role that the cation–π and cation–F interactions play in guiding coordination. Cation binding to uranyl is demonstrated in the solid state and in solution, providing the shortest reported crystallographic uranyl-oxo to potassium distance. UV-Vis, TD-DFT calculations, and electrochemical measurements show that cation coordination directly impacts the electronics at the uranium(VI) cation.

 Please wait while we load your content...
Please wait while we load your content...