Mechanistic insight into the ruthenium-catalysed anti-Markovnikov hydration of alkynes using a self-assembled complex: a crucial role for ligand-assisted proton shuttle processes†‡
Abstract
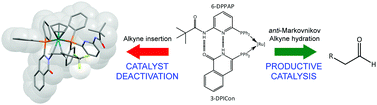
A combined computational and experimental study is presented that investigates the mechanism of the anti-Markovnikov hydration of phenylacetylene by [Ru(η5-C5H5)(6-DPPAP)(3-DPICon)]+ (where 6-DPPAP = 6-(diphenylphosphino)-N-pivaloyl-2-aminopyridine) and 3-DPICon = 3-diphenylphosphinoisoquinolone). The proposed mechanism, modelled using density functional calculations, involves an initial alkyne–vinylidene tautomerism, which occurs via a ligand-assisted proton shuttle (LAPS) mechanism. Intramolecular ligand assistance from the 6-DPPAP and 3-DPICon ligands, particularly the basic nitrogen of 6-DPPAP, is also involved in subsequent stages of the mechanism and three LAPS processes in total are observed. The self-assembled ligand backbone helps to create a water-binding pocket close to the metal centre, which facilitates nucleophilic attack of water at the vinylidene α-carbon and mediates protonation and deprotonation of subsequent acyl and vinyl intermediates. Experimental evidence is also presented for a novel non-productive catalyst deactivation pathway, which appears to arise from an initial lactam–lactim tautomerism of the 3-DPICon ligand followed by coupling with a vinylidene.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...