Recyclable chemosensor for oxalate based on bimetallic complexes of a dinucleating bis(iminopyridine) ligand†
Abstract
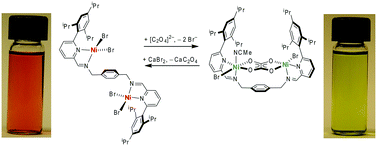
Herein we describe bimetallic di-nickel and di-copper complexes [Ni2(L)Br4] (1) and [Cu2(L)Br4(NCMe)2] (2) (L = (1E,1′E)-N,N′-(1,4-phenylenebis(methylene))bis(1-(6-(2,4,6-triisopropylphenyl)pyridin-2-yl)methanimine)) that bind oxalate intramolecularly to form [Ni2(L)Br2(C2O4)(NCMe)] (3) and [Cu2(L)Br2(C2O4)] (4). For the di-nickel complex 1, oxalate incorporation is accompanied by a significant colour change, from red-pink (1) to deep green (3). Mass spectrometric experiments demonstrate that the compound 1 is selective for oxalate versus related mono- and di-carboxylates tested. Oxalate can be released by the addition of slight excess of calcium bromide that forms insoluble calcium oxalate and restores the original Ni2(L)Br4 species. The product of the oxalate release was crystallized as [Ni2(L)Br4]·CaBr2(THF)4 species.

 Please wait while we load your content...
Please wait while we load your content...