Charge transfer processes: the role of optimized molecular orbitals
Abstract
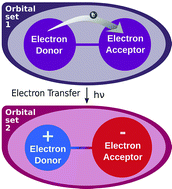
The influence of the molecular orbitals on charge transfer (CT) reactions is analyzed through wave function-based calculations. Characteristic CT processes in the organic radical 2,5-di-tert-butyl-6-oxophenalenoxyl linked with tetrathiafulvalene and the inorganic crystalline material LaMnO3 show that changes in the inner shells must be explicitly taken into account. Such electronic reorganization can lead to a reduction of the CT vertical transition energy up to 66%. A state-specific approach accessible through an adapted CASSCF (complete active space self-consistent field) methodology is capable of reaching good agreement with the experimental spectroscopy of CT processes. A partitioning of the relaxation energy in terms of valence- and inner-shells is offered and sheds light on their relative importance. This work paves the way to the intimate description of redox reactions using quantum chemistry methods.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...