Thermodynamics and high-pressure kinetics of a fast carbon dioxide fixation reaction by a (2,6-pyridinedicarboxamidato-hydroxo)nickel(ii) complex†
Abstract
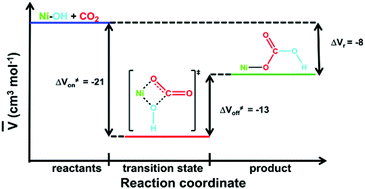
The previously reported carbon dioxide fixation reaction by the planar terminal hydroxide complex [Ni(pyN2Me2)(OH)]1− in DMF has been further characterized by determination of the equilibrium constants K298eq = 2.4 ± 0.2 × 105 M−1 and K223eq = 1.3 ± 0.1 × 107 M−1, as well as the volume of activation for the CO2 binding (ΔV≠223on = −21 ± 3 cm3 mol−1) and back decarboxylation (ΔV≠223off = −13 ± 1 cm3 mol−1) by high-pressure kinetics. The data are consistent with an earlier DFT computation, including the probable nature of the transition state, and support designating the reaction as one of the most completely investigated carbon dioxide fixation reactions of any type.

 Please wait while we load your content...
Please wait while we load your content...