Synthesis of γ-amidine-functionalized dianionic β-diketiminato lanthanide amides and trianionic β-diketiminato Na/Sm heterobimetallic complexes and their reactivity in polymerization of l-lactide†
Abstract
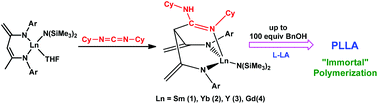
The readily accessible dianionic β-diketiminato lanthanide amido complexes LnLN(SiMe3)2(THF) (L = {(2,6-iPr2C6H3)NC(CH2)CHC(CH3)N(2,6-iPr2C6H3)}2−) show an unprecedented reactivity toward carbodiimides. The reaction with N,N′-dicyclohexylcarbodiimide (DCC) led via [4 + 2] cycloaddition to γ-amidine-functionalized dianionic β-diketiminato lanthanide amido complexes, LnL1N(SiMe3)2 (L1 = {[(NHC6H11)(NC6H11)C]HC[C(CH2)N(2,6-iPr2C6H3)]2}2−, Ln = Sm(1), Yb(2), Y(3), Gd(4)). Conversion of a mixture of SmLN(SiMe3)2(THF) and NaN(SiMe3)2 with carbodiimide furnished the heterobimetallic complexes of Sm/Na with a novel amidinate-functionalized trianionic β-diketiminate ligand, [Na(DME)2](μ-L2)[SmN(SiMe3)2] (L2 = {[C(NiPr)2]HC[C(CH2)N(2,6-iPr2C6H3)]2}3−, DME = dimethoxyethane) (5) for N,N′-diisopropylcarbodiimide (DIC) and [Na(DME)3]+[SmL3N(SiMe3)2]− (L3 = {[C(NCy)2]HC[C(CH2)N(2,6-iPr2C6H3)]2}3−) (6) for DCC. Molecular structures of complexes 1–6 were determined by an X-ray single crystal structure analysis. Complexes 1–4 were found to be highly active initiators of the ring-opening polymerization (ROP) of L-lactide (L-LA). The activity depended on the central metal with the increasing sequence of Yb < Y < Gd < Sm. Notably, the binary 1/BnOH (benzyl alcohol) system exhibited an “immortal” nature and proved able to convert 2000 equivalents of L-LA with up to 100 equivalents of BnOH per initiator. All the polylactides (PLAs) obtained showed monomodal, narrow molar mass distributions (Mw/Mn = 1.08–1.13) with the Mn (average number molar mass) decreasing with increasing amount of BnOH proportionally.

 Please wait while we load your content...
Please wait while we load your content...