N-heterocyclic carbenes as effective ligands for the preparation of stabilized copper- and silver-t-butylthiolate clusters†
Abstract
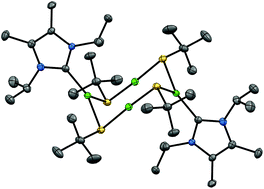
The ligation of N-heterocyclic carbenes (NHCs) to [CuStBu] and [AgStBu] was developed as an alternative to PR3 ligands as solubilizing reagents for these coordination polymers in order to form polynuclear copper and silver t-butylthiolate clusters. 1,3-Di-isopropylbenzimidazol-2-ylidene (iPr2-bimy) and 1,3-di-isopropyl-4,5-dimethylimidazol-2-ylidene (iPr2-mimy) were ligated to [CuStBu] and [AgStBu] forming [Cu4(StBu)4(iPr2-bimy)2] 1, [Cu4(StBu)4(iPr2-mimy)2] 2, [Ag4(StBu)4(iPr2-bimy)2] 5 and [Ag5(StBu)6][Ag(iPr2-mimy)2] 6. For comparison, the trialkyl phosphines PnPr3 and PiPr3 were also used to solubilize [AgStBu] and [CuStBu] to form copper and silver t-butylthiolate clusters. [Cu4(StBu)4(PnPr3)2] 3, [Cu4(StBu)4(PiPr3)2] 4, [Ag4(StBu)4(PnPr3)2] 7 and [Ag6(StBu)6(PiPr3)2] 8 were thus formed upon reaction with [CuStBu] and [AgStBu]. The synthesized complexes have been characterized via spectroscopic and crystallographic methods. The molecular structures of the clusters, which can vary according to the ligand type, are described.

 Please wait while we load your content...
Please wait while we load your content...