Theoretical studies of cyclic adenosine monophosphate dependent protein kinase: native enzyme and ground-state and transition-state analogues†
Abstract
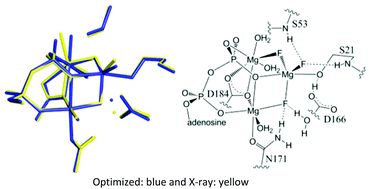
The mechanisms of phosphoryl transfer enzymes have garnered considerable attention. Cyclic AMP-dependent protein kinase (cAPK) catalyzes the transfer of the γ phosphoryl group of ATP to the serine hydroxyl group of a peptide chain. Metal-containing fluoro species have been used as transition-state and ground-state analogues in a variety of phosphoryl transfer enzymes and have shed light on the nature of the requirements in the active site to catalyze phosphoryl transfer. For cAPK, we present computational studies of the mechanism of phosphoryl transfer and the structure and 19F NMR spectra of various ground- (BeF3−) and transition-state (MgF3−, AlF4−, and AlF30) analogues. With native substrate, the phosphoryl transfer proceeds through a five-coordinate phosphorane transition state, i.e., there is not a five-coordinate phosphorane intermediate. Comparisons of simulated and experimental 19F NMR spectra show cAPK prefers a monoanionic analogue (MgF3− or AlF4−) over a neutral analogue (AlF3), supporting the charge balance hypothesis.

 Please wait while we load your content...
Please wait while we load your content...