Towards cancer cell-specific phototoxic organometallic rhenium(i) complexes†
Abstract
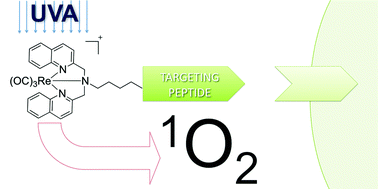
Over the recent years, several Re(I) organometallic compounds have been shown to be toxic to various cancer cell lines. However, these compounds lacked sufficient selectivity towards cancer tissues to be used as novel chemotherapeutic agents. In this study, we probe the potential of two known N,N-bis(quinolinoyl) Re(I) tricarbonyl complex derivatives, namely Re(I) tricarbonyl [N,N-bis(quinolin-2-ylmethyl)amino]-4-butane-1-amine (Re–NH2) and Re(I) tricarbonyl [N,N-bis(quinolin-2-ylmethyl)amino]-5-valeric acid (Re–COOH), as photodynamic therapy (PDT) photosensitizers. Re–NH2 and Re–COOH proved to be excellent singlet oxygen generators in a lipophilic environment with quantum yields of about 75%. Furthermore, we envisaged to improve the selectivity of Re–COOHvia conjugation to two types of peptides, namely a nuclear localization signal (NLS) and a derivative of the neuropeptide bombesin, to form Re–NLS and Re–Bombesin, respectively. Fluorescent microscopy on cervical cancer cells (HeLa) showed that the conjugation of Re–COOH to NLS significantly enhanced the compound's accumulation into the cell nucleus and more specifically into its nucleoli. Importantly, in view of PDT applications, the cytotoxicity of the Re complexes and their bioconjugates increased significantly upon light irradiation. In particular, Re–Bombesin was found to be at least 20-fold more toxic after light irradiation. DNA photo-cleavage studies demonstrated that all compounds damaged DNA via singlet oxygen and, to a minor extent, superoxide production.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...