Synthesis of a chiral fluorescence active probe and its application as an efficient catalyst in the asymmetric Friedel–Crafts alkylation of indole derivatives with nitroalkenes†
Abstract
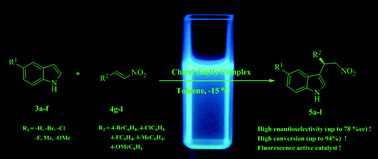
Novel chiral Zn(II) complexes were synthesized and these complexes showed fluorescence behaviour. These chiral Zn(II) complexes were tested in the asymmetric Friedel–Crafts alkylation reaction of indole derivatives with nitroalkenes. In all the cases, the desired product was obtained with high yield (up to 93%) and good enantioselectivity (up to 78% ee) under the optimized reaction conditions. The effects of solvent, metallic salt and piperidine ratio on the fluorescence intensity of the catalyst and on the enantioselectivity of the desired products were discussed.

 Please wait while we load your content...
Please wait while we load your content...