Regioselective catalytic acetoxylation of limonene
Abstract
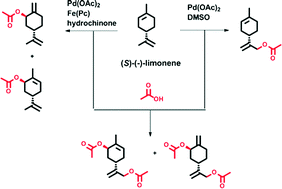
Two efficient strategies for a direct catalytic and regioselective acetoxylation of terpenes are described. Acetoxylated limonene derivatives were synthesized via palladium-catalyzed C–H activation utilizing para-benzoquinone (BQ) as reoxdidation agent and acetic acid as solvent and reactant. Addition of dimethyl sulfoxide (DMSO) to the catalytic system led to highly selective functionalization of the exocyclic double bond of limonene. This catalytic acetoxylation of limonene was further optimized with regard to a more sustainable and environmentally-friendly procedure. On the other hand, the use of an aerobic tandem catalytic system using iron(II) phthalocyanine (Fe(Pc)) as co-catalyst, which acts as electron transfer mediator (ETM), enabled a highly selective acetoxylation of the endocyclic double bond of limonene with high conversions. Moreover, diacetoxylated products were prepared by a reaction sequence applying the aforementioned catalytic systems.
- This article is part of the themed collection: Sustainable catalytic conversions of renewable substrates

 Please wait while we load your content...
Please wait while we load your content...