Design of nanostructures based on aromatic peptide amphiphiles
Abstract
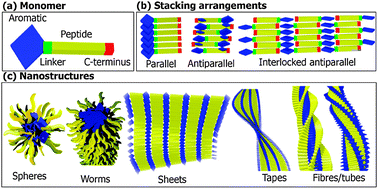
Aromatic peptide amphiphiles are gaining popularity as building blocks for the bottom-up fabrication of nanomaterials, including gels. These materials combine the simplicity of small molecules with the versatility of peptides, with a range of applications proposed in biomedicine, nanotechnology, food science, cosmetics, etc. Despite their simplicity, a wide range of self-assembly behaviours have been described. Due to varying conditions and protocols used, care should be taken when attempting to directly compare results from the literature. In this review, we rationalise the structural features which govern the self-assembly of aromatic peptide amphiphiles by focusing on four segments, (i) the N-terminal aromatic component, (ii) linker segment, (iii) peptide sequence, and (iv) C-terminus. It is clear that the molecular structure of these components significantly influences the self-assembly process and resultant supramolecular architectures. A number of modes of assembly have been proposed, including parallel, antiparallel, and interlocked antiparallel stacking conformations. In addition, the co-assembly arrangements of aromatic peptide amphiphiles are reviewed. Overall, this review elucidates the structural trends and design rules that underpin the field of aromatic peptide amphiphile assembly, paving the way to a more rational design of nanomaterials based on aromatic peptide amphiphiles.

 Please wait while we load your content...
Please wait while we load your content...