Transition-metal-free C–C bond forming reactions of aryl, alkenyl and alkynylboronic acids and their derivatives
Abstract
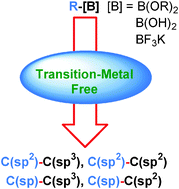
Investigation of new methods for the synthesis of C–C bonds is fundamental for the development of new organic drugs and materials. Aryl-, alkenyl- and alkynylboronic acids and their derivatives constitute attractive reagents towards this end, due to their stability, low toxicity and ease of handling. However, these compounds are only moderately nucleophilic. Consequently, the most popular C–C bond forming reactions of these boronic acids, such as the Suzuki–Miyaura, Heck, and Hayashi–Miyaura reactions, or additions to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, require catalysis by transition metals. However, due to the toxicity and cost of transition metals, some new methods for C–C bond formation using aryl-, alkenyl- and alkynylboronic acids under transition-metal-free conditions are beginning to emerge. In this tutorial review, the recent synthetic advances in this field are highlighted and discussed.
N bonds, require catalysis by transition metals. However, due to the toxicity and cost of transition metals, some new methods for C–C bond formation using aryl-, alkenyl- and alkynylboronic acids under transition-metal-free conditions are beginning to emerge. In this tutorial review, the recent synthetic advances in this field are highlighted and discussed.

 Please wait while we load your content...
Please wait while we load your content...