Applications of Bartoli indole synthesis
Abstract
In 1989, the reaction of vinyl magnesium halides with ortho-substituted nitroarenes leading to indoles was discovered. This reaction is now frequently reported as the “Bartoli reaction” or the “Bartoli indole synthesis” (BIS). It has rapidly become the shortest and most flexible route to 7-substituted indoles, because the classical indole syntheses generally fail in their preparation. The flexibility of the Bartoli reaction is great as it can be extended to heteroaromatic nitro derivatives and can be run on solid support. This review will focus on the use of the Bartoli indole synthesis as the key step in preparations of complex indoles, which appeared in the literature in the last few years.
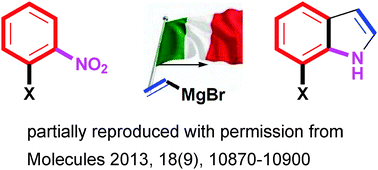

 Please wait while we load your content...
Please wait while we load your content...