Differences between amyloid-β aggregation in solution and on the membrane: insights into elucidation of the mechanistic details of Alzheimer's disease
Abstract
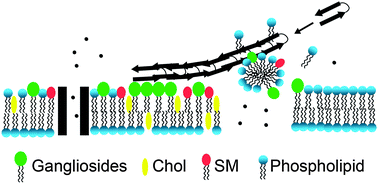
The association of the amyloid-β (Aβ) peptide with cellular membranes is hypothesized to be the underlying phenomenon of neurotoxicity in Alzheimer's disease. Misfolding of proteins and peptides, as is the case with Aβ, follows a progression from a monomeric state, through intermediates, ending at long, unbranched amyloid fibers. This tutorial review offers a perspective on the association of toxic Aβ structures with membranes as well as details of membrane-associated mechanisms of toxicity.
- This article is part of the themed collection: Molecular Medicine and Neurodegenerative Diseases

 Please wait while we load your content...
Please wait while we load your content...