Negative isotope effect for charge transport in acenes and derivatives – a theoretical conclusion†
Abstract
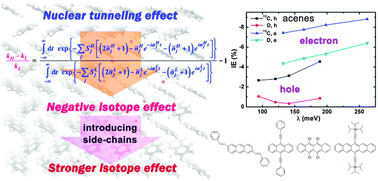
The isotope effect (IE) on charge transport in polyacenes was proposed in 1970 to judge the transport mechanism. However, there had not been a definitive answer for more than 40 years as to whether such an IE is positive or negative, both theoretically and experimentally, because either theory was too approximate or the experimental estimate was too rough to make a judgment. Employing the quantum nuclear tunneling model for organic semiconductors, we investigate the IE on both hole and electron transport for acenes and their derivatives. We show that both 13C-substitution and deuteration lead to a negative IE. By introducing phenyl, chlorine, or alkyl side-chains into acenes, the IE becomes more remarkable, especially for hole transport. The vibrational relaxation processes involving in-plane bending of ring or alkyl side-chain motions are found to be responsible for the IE.

 Please wait while we load your content...
Please wait while we load your content...