Formation of dibenzofuran, dibenzo-p-dioxin and their hydroxylated derivatives from catechol†
Abstract
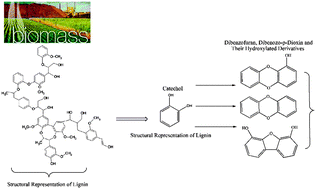
We present, in this study, mechanistic and kinetic accounts of the formation of dibenzofuran (DF), dibenzo-p-dioxin (DD) and their hydroxylated derivatives (OHs-DF/OHs-DD) from the catechol (CT) molecule, as a model compound for phenolic constituents in biomass. Self-condensation of two CT molecules produces predominantly a DD molecule via open- and closed-shell corridors. Coupling modes involving the o-semiquinone radical and the CT molecule (o-SQ/CT) generate two direct structural blocks for the formation of OHs-DF/OHs-DD structures, ether-type intermediates and di-keto moieties. The calculated reaction rate constants indicate that the fate of ether-type intermediates is to make hydroxylated diphenyl ethers rather than to undergo cyclisation reactions leading to the formation of preDF structures. Unimolecular loss of a H or OH moiety from a pivotal carbon in these hydroxylated diphenyl ethers then produces hydroxylated and non-hydroxylated DD molecules. Formation of OHs-DF initiated by o(C)–o(C) cross-linkages involving o-SQ/o-SQ and o-SQ/CT reactions incurs very similar reaction and activation enthalpies encountered in the formation of chlorinated DFs from chlorophenols.

 Please wait while we load your content...
Please wait while we load your content...