Photodegradation of 2-mercaptobenzothiazole and 1,2,3-benzotriazole corrosion inhibitors in aqueous solutions and organic solvents
Abstract
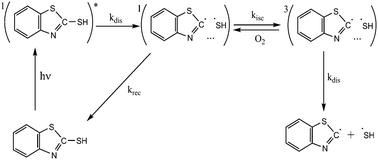
The photochemical degradation of 2-mercaptobenzothiazole (MBT) and 1,2,3-benzotriazole (BTA) inhibitors was studied in the present work in aqueous and in organic solutions. The extent of photodegradation was assessed by UV-Vis spectroscopy and the main reaction products were identified by tandem electrospray ionization mass spectrometry (ESI-MS/MS). The analysis of degradation products upon UV irradiation revealed the predominant formation of dimeric compounds from MBT and oligomeric structures from BTA, which were further converted into aniline. The increase of the quantum yield of MBT and BTA photodegradation reactions under aerobic conditions both in aqueous and organic solvents was explained by an increase of the spin–orbit conversion of the singlet radical pairs into the triplet radical pairs in the presence of oxygen. These triplet pairs further dissociate into free radicals, or convert to the parent compounds. At the early stage of UV irradiation, free radical coupling leads essentially to dimer formation in the case of MBT and to the formation of oligomers in the case of BTA irradiation.


 Please wait while we load your content...
Please wait while we load your content...