First-principles study of ground-state properties of U2Mo†
Abstract
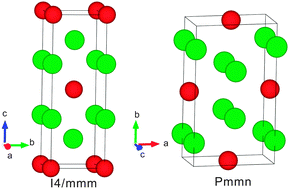
By means of first-principles calculations, we have systematically investigated the structural, elastic, vibrational, thermal and electronic properties of the ground-state phase for the intermetallic compound U2Mo. Our results reveal that the previously synthesized I4/mmm structure of U2Mo is a metastable phase and unstable, neither thermodynamically nor vibrationally at the ground state. In combination with the evolutionary structural searches, our first-principles calculations suggest a new ground-state Pmmn phase, which has been confirmed theoretically to be stable, both thermodynamically and vibrationally. Moreover, through the DFT + D technique we have discussed the influence of van der Waals interactions on the structural, elastic and vibrational properties, revealing a weak effect in pure U and Mo solids and U2Mo alloy. The analysis of the electronic band structures evidences its electronic stabilities with the appearance of a deep valley in the density of states at the Fermi level. Moreover, we have investigated further the temperature-dependent structural, thermal expansion and elastic properties of our proposed Pmmn ground-state phase. These results are expected to stimulate further experimental investigations of the ground-state phase of U2Mo.

 Please wait while we load your content...
Please wait while we load your content...