Nucleofugality in oxygen and nitrogen derived pseudohalides in Menshutkin reactions: the importance of the intrinsic barrier†‡
Abstract
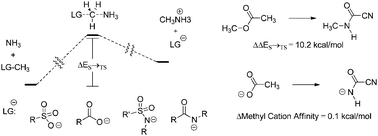
In order to study the nucleofugality of polyatomic anionic leaving groups derived from oxygen and nitrogen, a contingent of 19 methylating agents consisting of amines or alcohols activated with carbonyl or sulfonyl substituents has been examined via ab initio calculations. We have calculated gas phase activation energies for alkylation of ammonia, and methyl cation affinities. We find that polyatomic anionic leaving groups derived from nitrogen will have higher activation energies for Menshutkin (SN2) alkylation even when they have similar methyl cation affinities. This inherent deficit in the nucleofugality of nitrogen-derived leaving groups appears to be a result of the way bond cleavage is synchronized with bond formation to the incoming ammonia nucleophile. The nitrogen leaving groups showed greater dissociation from the methyl fragment than oxygen leaving groups relative to the length of the forming carbon–nitrogen bond. Additionally the second sulfonyl group present in a sulfonimide appears to be less effective at activating nitrogen due to a preference for tetrahedral geometries at the departing nitrogen in the transition states involving leaving sulfonamide groups. Optimal delocalization of electron density is therefore frustrated due to the geometry of the leaving group.

 Please wait while we load your content...
Please wait while we load your content...