Thermodynamic and kinetic characterization of transmembrane helix association†
Abstract
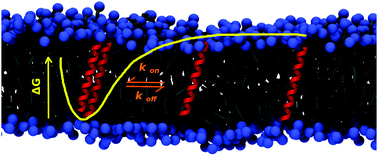
The transient dimerization of transmembrane proteins is an important event in several cellular processes and computational methods are being increasingly used to quantify their underlying energetics. Here, we probe the thermodynamics and kinetics of a simple transmembrane dimer to understand membrane protein association. A multi-step framework has been developed in which the dimerization profiles are calculated from coarse-grain molecular dynamics simulations, followed by meso-scale simulations using parameters calculated from the coarse-grain model. The calculated value of ΔGassoc is approx. −20 kJ mol−1 and is consistent between three methods. Interestingly, the meso-scale stochastic model reveals low dimer percentages at physiologically-relevant concentrations, despite a favorable ΔGassoc. We identify generic driving forces arising from the protein backbone and lipid bilayer and complementary factors, such as protein density, that govern self-interactions in membranes. Our results provide an important contribution in understanding membrane protein organization and linking molecular, nano-scale computational studies to meso-scale experimental data.

 Please wait while we load your content...
Please wait while we load your content...