Theoretical insights into nucleation of CO2 and CH4 hydrates for CO2 capture and storage
Abstract
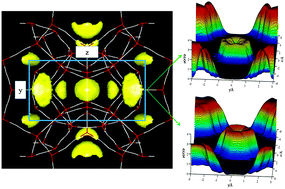
We present a hybrid three-dimensional (3D) theoretical approach, the density functional theory (DFT) integrated with the reference interaction site model (RISM), to investigate the nucleation of CO2 and CH4 hydrates. Within the theoretical framework, the 3D-RISM is applied to describe gas density distributions in hydrate cages, and the 3D-DFT is used to describe the interfacial structure and properties of the two hydrates, as well as their nucleation. The crystal–liquid phase equilibria of CO2 and CH4 hydrates are predicted by the hybrid 3D-DFT-RISM, and compared with the available experimental data to examine the theoretical model. In particular, the local and interfacial structure and properties, the critical nucleus radii and free-energy barriers at moderate concentration supersaturation are presented to analyze their nucleation. The formation enthalpies for the two hydrates are calculated to evaluate the possibility of CO2 storage by CH4–CO2 replacement in hydrate.

 Please wait while we load your content...
Please wait while we load your content...