Infrared detection of (H2O)20 isomers of exceptional stability: a drop-like and a face-sharing pentagonal prism cluster†
Abstract
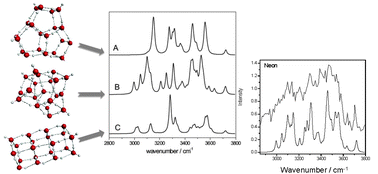
Water clusters with internally solvated water molecules are widespread models that mimic the local environment of the condensed phase. The appearance of stable (H2O)n cluster isomers having a fully coordinated interior molecule has been theoretically predicted to occur around the n = 20 size range. However, our current knowledge about the size regime in which those structures become energetically more stable has remained hypothetical from simulations in lieu of the absence of precisely size-resolved experimental measurements. Here we report size and isomer selective infrared (IR) spectra of (H2O)20 clusters tagged with a sodium atom by employing IR excitation modulated photoionization spectroscopy. The observed absorption patterns in the OH stretching region are consistent with the theoretically predicted spectra of two structurally distinct isomers of exceptional stability: a drop-like cluster with a fully coordinated (interior) water molecule and an edge-sharing pentagonal prism cluster in which all atoms are on the surface. The drop-like structure is the first experimentally detected water cluster exhibiting the local connectivity found in liquid water.

 Please wait while we load your content...
Please wait while we load your content...