Interactions between halide anions and interfacial water molecules in relation to the Jones–Ray effect
Abstract
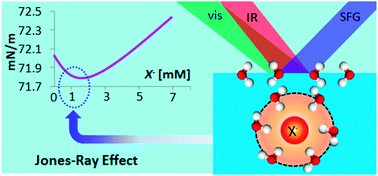
The Jones–Ray effect is shown to be governed by a different mechanism to enhanced anion adsorption. Halide ions at sub-molar concentrations are not exposed to the vapour phase; instead their first-solvating shell intimately interacts with the outmost water layer. Our novel proposal opens challenges for predicting related interfacial phenomena consistently.

 Please wait while we load your content...
Please wait while we load your content...