Molecular characterization of organic content of soot along the centerline of a coflow diffusion flame
Abstract
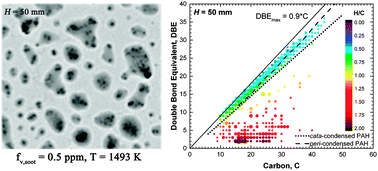
High-resolution mass spectrometry coupled with nanospray desorption electrospray ionization was used to probe chemical constituents of young soot particles sampled along the centerline of a coflow diffusion flame of a three-component Jet-A1 surrogate. In lower positions where particles are transparent to light extinction (λ = 632.8 nm), peri-condensed polycyclic aromatic hydrocarbons (PAHs) are found to be the major components of the particle material. These particles become enriched with aliphatic components as they grow in mass and size. Before carbonization occurs, the constituent species in young soot particles are aliphatic and aromatic compounds 200–600 amu in mass, some of which are oxygenated. Particles dominated by PAHs or mixtures of PAHs and aliphatics can exhibit liquid-like appearance observed by electron microscopy and be transparent to visible light. The variations in chemical composition observed here indicate that the molecular processes of soot formation in coflow diffusion flames may be more complex than previously thought. For example, the mass growth and enrichment of aliphatic components in an initial mostly aromatic structure region of the flame that is absent of H atoms or other free radicals indicates that there must exist at least another mechanism of soot mass growth in addition to the hydrogen abstraction–carbon addition mechanism currently considered in fundamental models of soot formation.

 Please wait while we load your content...
Please wait while we load your content...