Eutectic melting of LiBH4–KBH4†
Abstract
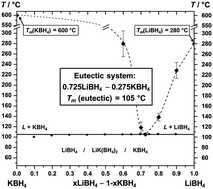
Eutectic melting in mixtures of alkali and alkali earth metal borohydrides can pave the way for new applications as fast ionic conductors, and facilitate hydrogen release by low temperature chemical reactions and convenient nanoconfinement. Here, we determine the eutectic composition for the lithium potassium borohydride system, 0.725LiBH4–0.275KBH4, with the lowest melting point, Tmelt ∼105 °C, of all known alkali and alkali earth metal borohydride mixtures. Mechanochemistry and manual mixing of LiBH4–KBH4 mixtures facilitate the formation of LiK(BH4)2. However, the melting or heat treatments used in this work do not produce LiK(BH4)2. The bimetallic borohydride dissociates into the monometallic borohydrides at ∼95 °C and partial melting occurs at ∼105 °C. Analysis of the unit cell volumes of LiBH4, KBH4 and LiK(BH4)2 in the temperature range 25 to 90 °C indicates that the formation of the bimetallic borohydride is facilitated by a more dense packing as compared to the reactants. Thus, LiK(BH4)2 is considered metastable and the formation is pressure induced. A phase diagram for the LiBH4–KBH4 system is established, which illustrates the low eutectic melting point and the stability range for the bimetallic borohydride, LiK(BH4)2.

 Please wait while we load your content...
Please wait while we load your content...