Impurity-related effects in poly(3-hexylthiophene) crystals
Abstract
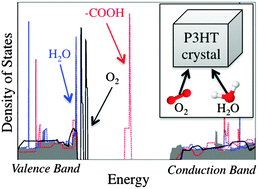
The performance of organic electronic devices often shows a strong dependence on the presence of impurities, for example oxygen and water molecules. Here we use first-principles calculations to examine oxygen- and water-related effects in poly(3-hexylthiophene) (P3HT), one of the most widely used donors in organic photovoltaics. We find that oxygen species may be stabilized in P3HT crystals in several different impurity configurations, including physisorbed and chemisorbed geometries. We also find that a number of these structures gives rise to levels inside the P3HT band gap and can thus act as carrier traps. In contrast, water molecules remain physisorbed between the polymer chains and induce only minimal changes in the electronic properties of the host system. The results are in agreement with pertinent experiments and elucidate the atomic-scale details of oxygen-related degradation of P3HT-based devices.

 Please wait while we load your content...
Please wait while we load your content...