Barrierless tautomerization of Criegee intermediates via acid catalysis†
Abstract
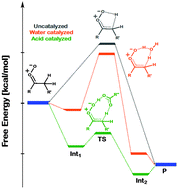
The tautomerization of Criegee intermediates via a 1,4 β-hydrogen atom transfer to yield a vinyl hydroperoxide has been examined in the absence and presence of carboxylic acids. Electronic structure calculations indicate that the organic acids catalyze the tautomerization reaction to such an extent that it becomes a barrierless process. In contrast, water produces only a nominal catalytic effect. Since organic acids are present in parts-per-billion concentrations in the troposphere, the present results suggest that the acid-catalyzed tautomerization, which can also result in formation of hydroxyl radicals, may be a significant pathway for Criegee intermediates.

 Please wait while we load your content...
Please wait while we load your content...