Electrochemical synthesis of copper nanoparticles using cuprous oxide as a precursor in choline chloride–urea deep eutectic solvent: nucleation and growth mechanism†
Abstract
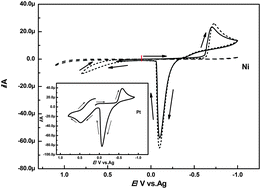
The electrochemical nucleation and growth kinetics of copper nanoparticles on a Ni electrode have been studied with cyclic voltammetry and chronoamperometry in the choline chloride (ChCl)–urea based deep eutectic solvent (DES). The copper source was introduced into the solvent by the dissolution of Cu(I) oxide (Cu2O). Cyclic voltammetry indicates that the electroreduction of Cu(I) species in the DES is a diffusion-controlled quasi-reversible process. The analysis of the chronoamperometric transient behavior during electrodeposition suggests that the deposition of copper on the Ni electrode at low temperatures follows a progressive nucleation and three-dimensional growth controlled by diffusion. The effect of temperature on the diffusion coefficient of Cu(I) species that is present in the solvent and electron transfer rate constant obeys the Arrhenius law, according to which the activation energies are estimated to be 49.20 and 21.72 kJ mol−1, respectively. The initial stage of morphological study demonstrates that both electrode potential and temperature play important roles in controlling the nucleation and growth kinetics of the nanocrystals during the electrodeposition process. Electrode potential is observed to affect mainly the nucleation process, whereas temperature makes a major contribution to the growth process.

 Please wait while we load your content...
Please wait while we load your content...