Interaction of insulin with anionic phospholipid (DPPG) vesicles
Abstract
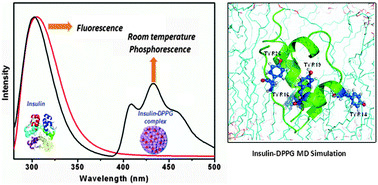
The interaction between a protein/enzyme and a lipid is critical for pharmacological activity. Here, we study the interaction between insulin and the 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol (DPPG) lipid anionic vesicle by successfully entrapping the insulin molecule into DPPG vesicles, which are biocompatible liposomes. For the insulin–DPPG complex system, steady state emission spectroscopy at room temperature (300 K) shows a new broad and structured peak between 400 nm and 500 nm along with the tyrosine fluorescence peak at 303 nm. Temperature dependent and time resolved spectroscopy reveal that the peak between 400 nm and 500 nm in the insulin–DPPG system arises due to the tyrosine phosphorescence phenomenon. This phosphorescence peak is the signature of insulin entrapment into the liposome. A molecular dynamics study of the tyrosine–DPPG system shows that the rigidity of tyrosine increases in the lipid layer. Dynamic light scattering (DLS), and zeta potential studies also establish the attachment of insulin with the anionic liposome.

 Please wait while we load your content...
Please wait while we load your content...