Interaction between the guanidinium cation and aromatic amino acids†
Abstract
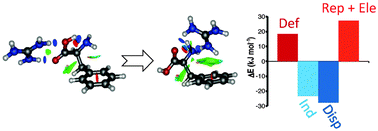
The interaction of the guanidinium cation with phenylalanine, tyrosine and tryptophan has been studied using a variety of computational methods. Benchmark values for the interaction have been estimated using the CCSD(T) method extrapolated to the complete basis set limit, indicating that the complexation energy amounts to −123.0, −124.4 and −134.2 kJ mol−1 for Phe, Tyr and Trp, respectively. Most stable minima correspond to neutral folded amino acids, with the cation interacting simultaneously with the carboxyl oxygen, the amino nitrogen and the aromatic ring. However, complexes with the amino acids as zwitterions are as stable as neutral ones. The final relative stability of the different structures results from a complex balance among different contributions to the complexation energy. Extended neutral structures are favored by larger electrostatic and smaller repulsion contributions, as well as by smaller deformation costs for bringing the amino acid to its final geometry into the complex. Zwitterions show large electrostatic and induction contributions that cancel out the huge deformation cost needed to transfer the proton to the amino group. The presence of the cation⋯π contact in folded minima introduces larger contributions from induction and dispersion (also as a consequence of the bulky guanidinium cation) that are able to overcome other effects, making folded minima the most stable together with zwitterionic ones.

 Please wait while we load your content...
Please wait while we load your content...