The enhancing effects of group V σ-hole interactions on the F⋯O halogen bond
Abstract
The σ-hole interaction, which occurs between the covalent IV–VII atoms and nucleophilic substances, has become a hot issue of weak interaction. In this work, NCF⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PX3⋯(NCF)n (X = F, Cl, Br, H, CH3; n = 0, 1, 2) complexes were constructed and studied based on the second-order Møller–Plesset perturbation theory (MP2) calculations to investigate the enhancing effects of group V σ-hole interactions on the F⋯O halogen bond. With increasing n, the F⋯O halogen bond becomes stronger, indicating that the group V σ-hole interactions could enhance the F⋯O halogen bond. As the capacity of donating electrons of X increases, the most negative electrostatic potentials outside the oxygen atom of O
PX3⋯(NCF)n (X = F, Cl, Br, H, CH3; n = 0, 1, 2) complexes were constructed and studied based on the second-order Møller–Plesset perturbation theory (MP2) calculations to investigate the enhancing effects of group V σ-hole interactions on the F⋯O halogen bond. With increasing n, the F⋯O halogen bond becomes stronger, indicating that the group V σ-hole interactions could enhance the F⋯O halogen bond. As the capacity of donating electrons of X increases, the most negative electrostatic potentials outside the oxygen atom of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PX3⋯(NCF)n (n = 0, 1, 2) become more negative, resulting in a stronger F⋯O halogen bond. In the formation of a F⋯O halogen bond, along the sequence of X = F, Cl, Br, H, CH3 of the negative sites O
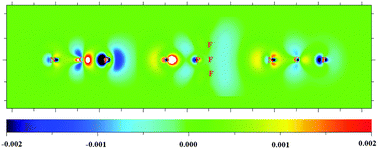
PX3⋯(NCF)n (n = 0, 1, 2) become more negative, resulting in a stronger F⋯O halogen bond. In the formation of a F⋯O halogen bond, along the sequence of X = F, Cl, Br, H, CH3 of the negative sites O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PX3, the electric field of the lone pair of oxygen becomes greater and causes a larger decrease in electron density outside the fluorine atom. On the other hand, with increasing n from 0 to 2, the group V σ-hole interactions also increase the electric field of lone pair of oxygen and results in a larger decrease in electron density outside the fluorine atom.
PX3, the electric field of the lone pair of oxygen becomes greater and causes a larger decrease in electron density outside the fluorine atom. On the other hand, with increasing n from 0 to 2, the group V σ-hole interactions also increase the electric field of lone pair of oxygen and results in a larger decrease in electron density outside the fluorine atom.

 Please wait while we load your content...
Please wait while we load your content...