A DFT study on the reaction mechanism of dimerization of methyl methacrylate catalyzed by N-heterocyclic carbene†
Abstract
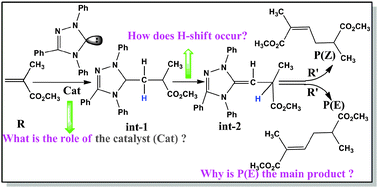
Reaction mechanisms of the N-heterocyclic carbene (NHC)-catalyzed dimerization of methyl methacrylate were studied using density functional theory (DFT) at the M05-2X/6-31G(d,p) level of theory. Four possible reaction channels (A, B, C, and D) have been investigated in this work. Particularly, we proposed a novel reaction pathway, where the proton transfers are assisted by a different molecule. The calculated results indicate that the channels B and D are more energetically favourable channels. The obtained results suggest that the E-isomer product is the main product, which is in agreement with the experimental results. Further calculations and analyses of global and local reactivity indices reveal the role of the NHC catalysts in the title reaction. The mechanistic insights gained are valuable for not only rational design of more efficient NHC catalysts but also for understanding the similar reaction mechanism.

 Please wait while we load your content...
Please wait while we load your content...