From two-dimension to one-dimension: the curvature effect of silicon-doped graphene and carbon nanotubes for oxygen reduction reaction
Abstract
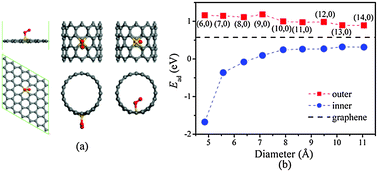
For the goal of practical industrial development of fuel cells, inexpensive, sustainable, and highly efficient electrocatalysts for oxygen reduction reactions (ORR) are highly desirable alternatives to platinum (Pt) and other rare metals. In this work, based on density functional theory, silicon (Si)-doped carbon nanotubes (CNTs) and graphene as metal-free, low cost, and high-performance electrocatalysts for ORR are studied systematically. It is found that the curvature effect plays an important role in the adsorption and reduction of oxygen. The adsorption of O2 becomes weaker as the curvature varies from positive values (outside CNTs) to negative values (inside CNTs). The free energy change of the rate-determining step of ORR on the concave inner surface of Si-doped CNTs is smaller than that on the counterpart of Si-doped graphene, while that on the convex outer surface of Si-doped CNTs is larger than that on Si-doped graphene. Uncovering this new ORR mechanism on silicon-doped carbon electrodes is significant as the same principle could be applied to the development of various other metal-free efficient ORR catalysts for fuel cell applications.

 Please wait while we load your content...
Please wait while we load your content...