CO oxidation catalyzed by Pt-embedded graphene: a first-principles investigation
Abstract
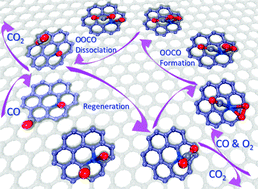
We addressed the potential catalytic role of Pt-embedded graphene in CO oxidation by first-principles-based calculations. We showed that the combination of highly reactive Pt atoms and defects over graphene makes the Pt-embedded graphene a superior mono-dispersed atomic catalyst for CO oxidation. The binding energy of a single Pt atom onto monovacancy defects is up to −7.10 eV, which not only ensures the high stability of the embedded Pt atom, but also vigorously excludes the possibility of diffusion and aggregation of embedded Pt atoms. This strong interfacial interaction also tunes the energy level of Pt-d states for the activation of O2, and promotes the formation and dissociation of the peroxide-like intermediate. The catalytic cycle of CO oxidation is initiated through the Langmuir–Hinshelwood mechanism, with the formation of a peroxide-like intermediate by the coadsorbed CO and O2, by the dissociation of which the CO2 molecule and an adsorbed O atom are formed. Then, another gaseous CO will react with the remnant O atom and make the embedded Pt atom available for the subsequent reaction. The calculated energy barriers for the formation and dissociation of the peroxide-like intermediate are as low as 0.33 and 0.15 eV, respectively, while that for the regeneration of the embedded Pt atom is 0.46 eV, indicating the potential high catalytic performance of Pt-embedded graphene for low temperature CO oxidation.

 Please wait while we load your content...
Please wait while we load your content...