Electrochemical flow-based solution–solid growth of the Cu2O nanorod array: potential application to lithium ion batteries†
Abstract
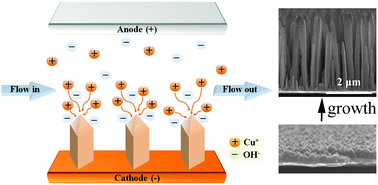
The catalyzed solution–liquid–solid (SLS) growth has been well developed to synthesize semiconductor nanowires with controlled diameters. The SLS growth occurs in the longitudinal direction of nanowires, due to the directional anisotropy driven by the metal catalysts where chemical precursors are introduced. In the present study, we report a selective, template-free, and environmentally-friendly electrochemical flow-based solution–solid (electrochemical flow-SS) growth of the Cu2O nanorod array. The anisotropy for directional growth without any catalysts is generated by the electrical field in a flowing electrolyte of ultra-dilute CuSO4. The filamentary anisotropy originates from electric field enhancement on pyramidal nanocrystals in the electrolyte of low ionic conductivity (13 μS cm−1). The Cu2O and Cu nanorods are able to be selectively synthesized by controlling the electrolyte pH and oxygen dissolution into the electrolyte. The synthesized Cu2O nanorod array shows excellent electrochemical properties as an anode material for lithium-ion batteries; the specific capacities increase from 323 to 1206 mA h g−1 during 500 cycles. The capacity enhancement is due to the phase transformation from Cu2O to CuO, nano-restructuring of nanorods into fragmented nanoparticles, and the progressive generation of an electroactive polymeric gel-like layer on the surface of the nanoparticles. The electrochemical flow-SS growth of Cu2O nanorods is expected to contribute to further development of other functional nanorods.

 Please wait while we load your content...
Please wait while we load your content...