Nanoparticle impacts reveal magnetic field induced agglomeration and reduced dissolution rates†
Abstract
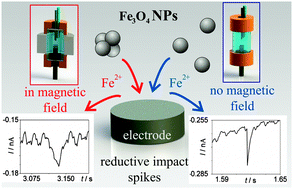
Superparamagnetic nanoparticles (NPs) are used in a variety of magnetic field-assisted chemical and medical applications, yet little of their fate during magnetic field interrogation is known. Here, fundamental and new insights in this are gained by cathodic particle coulometry. This methodology is used to study individual Fe3O4 NPs in the presence and absence of a magnetic field. It is first noticed that no major NP agglomeration occurs in the absence of a magnetic field even in a suspension of high ionic strength. In contrast, a significant magnetic field-induced agglomeration of NPs is observed in a magnetic field. A second new finding is that the dissolution of Fe3O4 NPs is strongly inhibited in a magnetic field. This is explained as a result of the magnetic field gradient force trapping the released Fe2+ ions near the surface of a magnetized Fe3O4 NP and thus hindering the mass-transport controlled NP dissolution. Consequently, fundamental magnetic field effects are measured and quantified on both the single NP scale and in suspension and two novel effects are discovered.

 Please wait while we load your content...
Please wait while we load your content...